Abstract
BACKGROUND
Bortezomib-based induction (V-IND) approaches are used in >90% of Australian newly diagnosed transplant eligible multiple myeloma (NDTE MM) patients (pts) with a maximum of 4 cycles of V-IND therapy available via the pharmaceutical benefits scheme (PBS) prior to a planned autologous stem cell transplantation (ASCT). However, NDTE MM patients failing V-IND (defined as best response < partial response [PR]) demonstrate shortened survival and continue to represent a sub-group of MM where a clear unmet medical need persists. The ALLG MM21 was designed to evaluate the efficacy of an early response adapted approach with a switch to an intensive Daratumumab-lenalidomide-dexamethasone (DRd)-based salvage-ASCT- consolidation strategy in patients failing V-IND.
METHOD
We present the results of a planned interim analysis of the multi-centre single arm study MM21 (ACTRN12618001490268). Eligible pts were NDTE MM who had received V-IND pre-ASCT and demonstrated either a sub-optimal response (SOR - defined as <minimal response [MR] after 2 cycles or <PR after 4 cycles of V-IND) or primary refractoriness (1REF - defined as disease progression while on or within 60 days of completing V-IND). Pre-ASCT DRd was DARA 16mg/kg IV days 1, 8, 15 and 22 for cycles 1 (C1) and 2, and on days 1 and 15 of C3 and C4; Lenalidomide 25mg OD D1-21; and, dexamethasone 40mg PO on D 1, 8, 15 and 22 of each 28-day cycle for C1 to C4. Anti-thrombotic and anti-viral prophylaxis was as per individual institutional practice. Between C3 and C4, patients underwent a G-CSF mobilised PBSC collection with a melphalan 200mg/m2 conditioned ACST after C4. Patients underwent D100 post-ASCT disease response assessment including EuroFlow minimal residual disease (MRD) testing. In the absence of disease progression, patients then received 12, 28-day cycles of consolidation comprising DARA IV 16mg/kg on D1, 15 of C1 and C2 and on D1 of C3 to C12, lenalidomide 25mg PO on D1-21 of C1 and C2 and 10mg OD on days 1-28 of C3 to C12; dexamethasone 40mg was weekly from C1 to C12.
RESULTS
Fifty patients were recruited from 7 Australian sites between March 2019 and July 2020. Median age was 61 years with 66% males. Disease status at study entry was SOR in 72% (<MR n = 9, <PR n = 27) and 1REF in 28%. Data cut-off date was June 30 2021. 45 patients (90%) received 4 complete cycles of salvage DRd. 11/50 (22%) patients did not undergo ASCT and 4 patients failed stem cell collection. Two pts were withdrawn due to treatment related gastrointestinal toxicity - persistent oesophagitis (n =1) and recurrent colitis (n=1). There were two deaths, due to COVID-19 pneumonia (n =1) and septic shock (n =1).
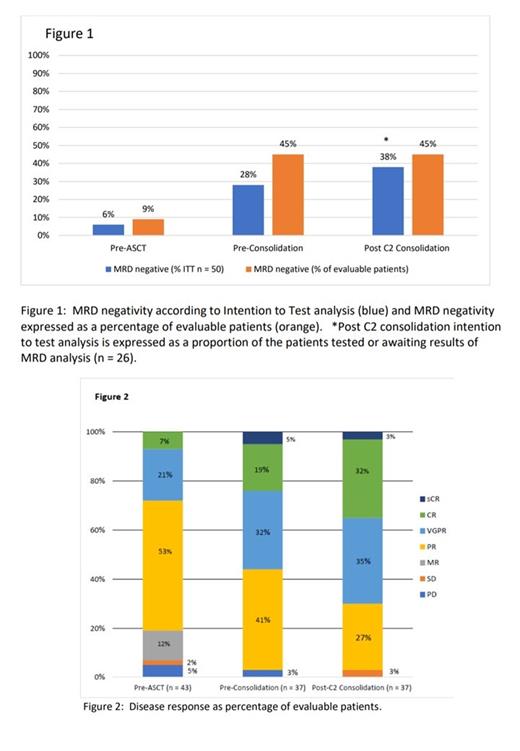
Pre-ASCT response was evaluable in 43 patients, overall response rate (ORR) was 70% - complete response (CR) 6%, very good partial response (VGPR) 18%, partial response (PR) 46%, clinical benefit rate (CBR) 83% - MR 11% and stable disease (SD) 2% on Intention to Treat (ITT n = 50) analysis. 33 patients were assessed for MRD - MRD negative 6% on ITT (3/33 9%).
Pre-consolidation disease assessment was evaluable in 37 pts, both ORR and CBR were 72% - stringent complete response (sCR) 4%, CR 14%, VGPR 24%, PR 30% ITT analysis. 31 pts were evaluated for MRD - MRD negative 28% ITT (14/31 45%). In 6 patients, MRD was omitted or could not be performed due to pre-analytical issues.
Post-C2 consolidation assessment was evaluable in 37 pts, ORR 72% - sCR 2%, CR 24%, VGPR 26%, PR 20%, CBR 74% - SD 2% ITT analysis. To date, 22 patients have been evaluated for MRD with 4 patients awaiting results, MRD negative rate of 38% ITT (10/22 45%). MRD sample collection at this time-point was omitted in 7 patients, potentially skewing MRD negativity on ITT analysis.
CONCLUSION
Preliminary analysis of the MM21 trial demonstrates early response-adaptive escalation to DRd facilitated ASCT in the majority patients with robust ORR post-autologous stem cell transplant and substantial improvement in disease control, as reflected in improved rates of MRD and disease response to treatment. At both post-ASCT time-points there was significant drop off in MRD testing due to testing omission, potential skewing results of MRD analysis. MRD and duration of response analysis following C12 consolidation is planned and will be of interest. Current data suggests this drug combination shows potential for substantial benefit in the study population.
Lim: BMS: Honoraria; Janssen-Cilag: Honoraria; Amgen: Honoraria. Reynolds: Abbvie: Research Funding; Alcon: Current equity holder in publicly-traded company; Novartis AG: Current equity holder in publicly-traded company. Quach: Janssen/Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Estell: Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Janowski: Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Kalff: Sandoz: Honoraria; CSL: Honoraria; Roche: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria. Spencer: Takeda: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding; BMS: Research Funding; Janssen-Cilag: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; STA: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal